Use a formula that relates molecular mass, density, pressure, temperature, and the constant R.
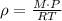
Using the given information, we have
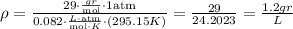
The density is 1.2 grams per liter.
Then, use the density formula to find the mass.
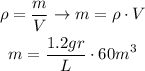
But, 1 liter equals 0.001 m^3 and 1kg equals 1000gr.
![\begin{gathered} m=\frac{1.2gr\cdot\frac{1\operatorname{kg}}{1000gr}}{1L\cdot(0.001m^3)/(1L)}\cdot60m^3=(0.072)/(0.001)kg \\ m=72\operatorname{kg} \end{gathered}]()
Therefore, the answer is c. 72 kg.