Since we want the calories to biol, we need the heat of vaporization, which is give to be 540.0 cal/g.
We have two formulas for heat, one is for when we are not changing phase and we are changing temperature:
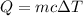
And the other is for when we are not changing temperature and we are changing phase:
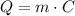
In this case, we are changing only phase, because the water is boiling in its vaporization temperature, that is, it is going from liquid to gas, so we need to use the second equation.
In this equation, we have: Q is the heat needed, m is the mass and C is the heat of vaporization.
So:
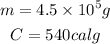
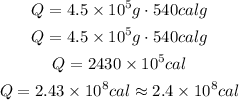
So, the heat needed is 2.43 x 10⁸ cal or approximately 2.4 x 10⁸ cal.