Answer:
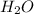
Step-by-step explanation:
Here, we want to get the type of substances which are acids according to the Bronsted-Lowry definition
According to their definition, an acid donates or is the source of H+ ions in an acid-base reaction
Let us look at the reactants, we can see that while the ammonia molecule accepted a donation of hydrogen ion to become Ammonium ion, the water molecule lost a hydrogen ion to become the hydroxide ion
Thus, we can conclude here that the water molecule is the acid in the reaction