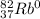To write its complete symbol representation, we need:
- its charge
- the atomic number (which gives its symbol)
- its mass number
The charge is given since it says that it is neutral. A neutral atom has charge 0.
The mass number is given, 80. The mass number is the sum of the atomic numeber and the number of neutrons, which is 45. Thus, the atomic number, z, is:
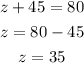
Checking a periodic table, we see that the element with atomic number is bromine, which has the symbol Br.
Now, we just need to write the symbolic representation. It has the symbol Br in the middle, the atomic number, 35, in the left subsbript, the mass number, 80, in the left superscript, and the charge, 0, in the right superscript.
So, the representation is:
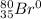
Since this other atom has 2 more protons and two more electrons, the atomic number will go from 35 to 37, the mass number will go from 80 to 82. Checking the periodic table, we see that the element with this atomic number is Rubidium, with symbol Rb.
The charge is the difference between protons and electrons. Since the first atom is neutral, it has the same number of protons and electrons, 35, so the charge is 35 - 35 = 0.
Since we have 2 more protons and 2 more electrons, we have 37 of each, so the charge wil still be neutral, because 37 - 37 = 0.
So, we have:
- Charge: 0
- Atromic number: 37
- Symbol: Rb
- Mass number: 82.
So, the representation is: