Find the kinetic energy of the electron after moving through the potential difference of 702.06kV.
The electric potential energy of a charge is transformed in kinetic energy when it passes through a potential difference V. Then, the kinetic energy of the electron is:
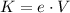
Where e is the charge of an electron:
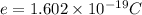
On the other hand, the kinetic energy of a particle with mass m is related to its speed:
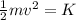
In this case, m is the mass of the electron:
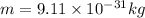
Then:
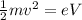
Isolate the speed v from the equation:
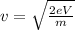
Replace the values for e, m and V=702.06kV to find the speed of the electron:

Notice that the speed of the electron calculated using the classical method is greater than the speed of light:
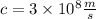
Then, the theory of Special Relativity is required to find a proper answer for this problem.
Therefore, the (inadequate) answer is: the electron is moving at a speed of approximately 4.97*10^8m/s according to Classical Physics.