ANSWER :
272.2 mL of 65% dextrose solution
EXPLANATION :
From the problem, we have to concentrations, 20% and 65% dextrose.
Let x = amount of 20% dextrose
y = amount of 65% dextrose
The sum of x and y is 490 mL, since it is the volume or space of the IV bag.
So we have :
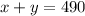
The resulting concentration must be 45%, so that will be :
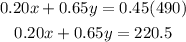
Now we have two equations two unknowns.
Express the first equation as x in terms of y :
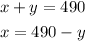
Substitute x to the second equation :
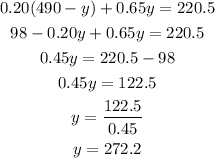
So we have y = 272.2 mL.
It needs 272.2 mL of 65% dextrose solution.