We will firstly start by writing a balanced chemical equation for the reaction:
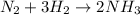
We will determine the limiting reactant:
Given we have 4.0 moles of nitrogen we will determine the amount of moles of hydrogen that is needed to react with the nitrogen. We will do the same for hydrogen, if we have 9.0 moles of hydrogen gass how many moles of nitrogen is needed to react with the hydrogen.
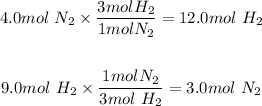
Findings:
a) For 4.0 moles of nitrogen it will take 12.0 moles of hydrogen but we only have 9.0 moles of hydrogen.
b) For 9.0 moles of hydrogen it will require 3 moles of nitrogen and we have 4.0 moles.
c) With this information we see that hydrogen is the limiting reactant because it is the first reactant we run out of.
We will now use the limiting reactant to calculate the theoretical yield because the limiting reactant determine the amount of product that is formed.
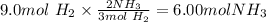
The percent yield is given by:

Answer: The percent yield for the reaction is 50%.