Answer:
14 moles of O2.
Step-by-step explanation:
You can see that in the chemical reaction, 2 moles of O2 reacted, produces 2 moles of H2O. You can realize that the molar ratio between these two compounds is 1:1, this means that if we react 2 moles of oxygen with CH4, we will produce 2 moles of H2O, or if we want to react 5 moles of oxygen with CH4, we will produce 5 moles of H2O. You can see this better, like this:
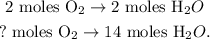
The calculation of this rule of three can be visualized:
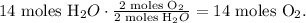
We need 14 moles of O2 to produce 14 moles of H2O.