INFORMATION:
We know that:
- the gas occupies 0.59L and has a pressure of 752 mm Hg at 36 Celsius
And we must find the number of moles of the gas
STEP BY STEP EXPLANATION:
To find the number of moles we need to use the ideal gas formula

So, we must solve it for n
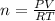
Now, the given information is
- Pressure (P) = 752 mmHg
- Volume (V) = 0.59 L
- Temperature (T) = 36°C
We can use R = 62.36 (mmHg * L)/(mol * K)
So, we must convert the temperature to K
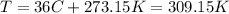
Finally, replacing the values in the formula
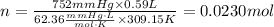
0.0230 moles of gas are contained in a human breath that occupies 0.59L and has a pressure of 752 mm Hg at 36 Celsius.
ANSWER:
0.0230 mol