Step 1 - Discovering the density of the mixture
Since we know the density of each component of the mixture (ISA propanol and water) and we know the volume of each one, we can calculate the mass of each component by the following relation:
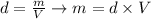
Calculating the mass of each component, we obtain:
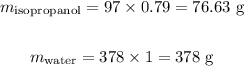
The total mass of the mixture will be thus:

Now, to obtain the density of the mixture, let's divide the total mass by the total volume, i.e., 451 ml:
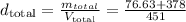
Step 2 - Discovering the percent volume of isopropanol
You probably have noted we did not sum 76.63 and 378 together. There's a reason for this: we want to keep them separate so as to disocover the density of each substance in the mixture. We'll obtain this by applying the folowing propertie of division:
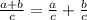
Using this propertie we can write that:
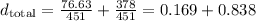
We have obtained thus the "new" densities of isopropanol (0.169 g/ml) and water (0.838 g/ml) in the mixture. We know, therefore, that in each ml of mixture we have 0.169 g of isopropanol.
We can convert this mass to volume by using again the following relation:
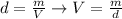
For isopropanol, d = 0.79 g/ml and m = 0.169 g, therefore:
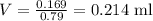
Since this is the volume of isopropanol is contained in 1 ml of solution, the volume percent can by found by setting the following proportion:
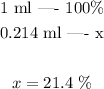
The volume percentage of isopropanol is thus 21.4 %.