Step 1 - Discovering the total concentration of vitamin c
The molar concentration can be obtained by dividing the number of moles by the volume:
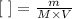
In this equation, m represents the mass, M the molar mass and V the volume. For vitamin c, we have:
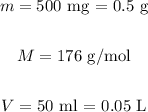
Substituting these values on the equation above:
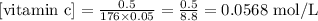
Step 2 - Finding the concentration of H+
Vitamin c is a monoprotic acid (I'll represent it by VitcH), and it will react with water to form H3O+:
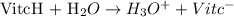
The concentration of H3O+ that came from the ionization of vitamin c can be calculated by the pH:
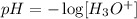
Since pH=2.67, we have:
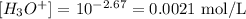
Step 3 - Finding the percent ionization
Now that we know how many vitamin c was dissolved and how many of it was ionized to form H3O+, we can set the following proportion:
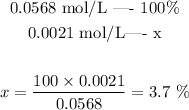
The percent ionization is thus 3.7 %. Note that Vitamin c is a very weak acid.