Answer: the percent composition of Ba(OH)2 is: 80.15% Ba, 18.67% O and 1.176% H
Step-by-step explanation:
The question requires us to determine the percent composition of Ba(OH)2.
The percent composition of a compound describes the amount of each element in the molecule in terms of mass percentage. We can determine the percentage composition using the molar mass of the compound and the molar masses of each element, considering the amount of atoms of these elements in the compound.
1) Determining the molar mass of the compound
The atomic masses of Ba, O and H are as it follows:
atomic mass Ba = 137.3 u
atomic mass O = 15.99 u
atomic mass H = 1.007 u
To calculate the molar mass of Ba(OH)2, we'll need to consider the amount of each element in the molecule and the atomic mass of the elements:
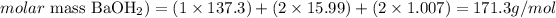
Therefore, the molar mass of Ba(OH)2 is 171.3 g/mol.
2) Determining the percent composition
We can determine the percent composition of each element in the compound using the following equation:
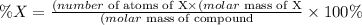
where X is a generic element.
Thus, considering the molar mass calculated and the atomic masses listed previously, we can calculate the percent composition of Ba, O and H in the molecule Ba(OH)2 as:
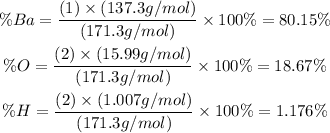
(note that the atomic mass, in u or amu, is numerically equal to the molar mass of the element, in g/mol).
Therefore, the percent composition of Ba(OH)2 is: 80.15% Ba, 18.67% O and 1.176% H.