Given:
Temperature of hot water, T1 = 48°C
Temperature of cold water, T2 = 12°C
Final temperature = 36 °C
Total mass of water = 90 kg
Let's find the mass of hot water.
Let x represent the mass of hot water.
We have:
Mass of hot water = x
Mass of cold water = 90 - x
• Fall in temperature of hot water, ΔT1 = T1 - T = 48°C - 36 °C = 12°C
• Temperature increase of cold water, ΔT2 = 36 °C - 12°C = 24°C.
Apply the principle of calorimetry:
Heat gained by cold water = Heat lost by water
Thus, we have:
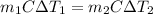
Where:
m1 is the mass of hot water
m2 is the mass of cold water.
Thus, we have:
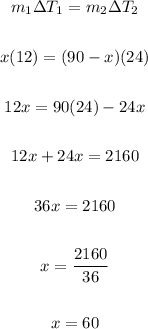
Therefore, the mass of hot water you must mix is 60 kilograms.
ANSWER:
60 kg