Answer:
The temperature is 2,231.36 K. (The closest value is 2,232.35K).
Step-by-step explanation:
The given information from the exercise is:
- Initial temperature (T1): 80°C (353K)
- Initial volume (V1): 38.6mL
- Final volume (V2): 244mL
1st) It is necessary to convert the temperature unit from °C to K:
80+273 = 353K
2nd) Now we can calculate the final temperature (T2), by replacing the values of T1, V1 and V2 in the Charles's law formula:
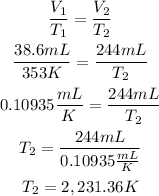
So, the temperature is 2,231.36 K. (The closest value is 2,232.35K).