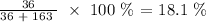Answer:
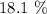
Step-by-step explanation:
Here, we want to get the percentage of water by mass in the given hydrate
What we have to do here is to divide the molar mass of the hydrate part by the molar mass of the whole compound and write as a percentage
Mathematically, we have the molar mass of the water part as 2(18 g/mol) = 36 g/mol (1 mole of water has a mass of 36 g)
For the other part which is FeCl3, we have the molar mass as 163 g/mol
Thus, we have the percentage by mass of water as: