Answer:
Step-by-step explanation:
1st) It is necessary to calculate the molar mass of NH4OH:
- N atomic mass: 14 g/mol
- H atomic mass: 1.01 g/mol
- O atomic mass: 15.99 g/mol
We have to multiply the atomic mass of each element by its subscript in the NH4OH formula:
- N atomic mass x 1 = 14 g/mol
- H atomic mass x 5 = 5.05 g/mol (in this case the total number of H in the molecule is 5)
- O atomic mass x 1 = 15.99 g/mol
Now we have to add all:
NH4OH molar mass = 14 g/mol + 5.05 g/mol + 15.99 g/mol
NH4OH molar mass = 35.04 g/mol
2nd) Finally, we can calculate the mass percent of nitrogen in NH4OH, assuming that the molar mass of NH4OH (35.04g/mol) is the 100%. It is necessary to use the total amount of nitrogen in the NH4 molecule (14 g):
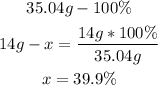
So, the mass percent composition of nitrogen is 39.9%.