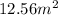Question:
Solution:
Circumference of the circle:
The Circumference C of a circle of radius r is given by the following formula:
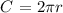
in this case r=2m, then the circumference would be:
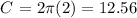
that is, the circumference of the circle is:
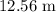
Area of the circle:
The area A of a circle of radius r, is given by the following formula:
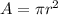
According to the data of the problem, we can conclude that the area of the given circle is:
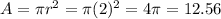
that is, the area of the given circle is: