Answer:
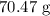
Step-by-step explanation:
Here, we want to get the mass of the copper metal produced
From what we have in the balanced equation of reaction, the mole ratio for all is 1 to 1
This is the theoretical mole ratio
To get the actual, we need to look through all
Firstly, let us get the mass produced considering zinc
We need to get the number of moles produced
That would be the mass of zinc divided by the atomic mass of zinc
The atomic mass of zinc is 65 amu
The number of moles is thus: 139/65 = 2.1385 moles
The mass of copper would be the this number of moles multiplied by the atomic mass of copper
The atomic mass of copper is 64 amu
The mass is thus:
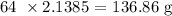
Now, let us do the same considering copper ii nitrate
We divide the mass by the molar mass
The molar mass of copper ii nitrate is 188 g/mol
The number of moles will be:
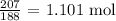
The mass of copper produced will be the product of the number of moles and the atomic mass of copper. The atomic mass of copper is 64 amu
The mass is thus:
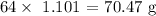
Copper nitrate produces less amount of copper and thus it is the limiting reagent and its value should be considered