ANSWER
The actual yield of aluminum chloride is 25.49 gm
The theoretical yield of aluminum chloride is 48.98 gm
Explanation
Given information
The mass of aluminum = 34.0 gm
The mass of chlorine = 39.0 gm
The next step is to write down the balanced chemical reaction
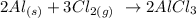
The next step is to find the moles of aluminum and chlorine using the below formula
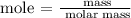
According to the periodic table, the molar mass of aluminum is 26.981 g/mol and the molar mass of chlorine is 35.5 g/mol

The next step is to determine the limiting reagent from the number of moles by dividing the moles by the coefficient
From the reaction, you will see that aluminum has 2 moles and chlorine has 3 moles

From the calculations above, you will see that chlorine has the least number of moles, and it is the limiting reagent
Since the limiting reagent is chlorine, then, we can now find the number of moles of aluminum chloride using a stoichiometry ratio
From the balanced reaction, 3 moles of chlorine yield 2 moles of aluminum chloride.
Let x be the number of moles of aluminum chloride
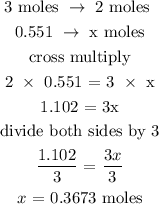
Therefore, the number of moles of aluminum chloride is 0.3673 moles
The next step is to find the mass of aluminum chloride using the below formula
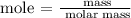
According to the periodic table, the molar mass of aluminum chloride is 133.34 g/mol

Therefore, the theoretical yield of aluminum chloride is 48.98 gm
The next step is to find the actual yield of the aluminum chloride by using the below formula
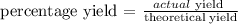
Recall that, the percentage yield is 51%
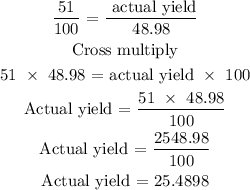
Therefore, the actual yield of the aluminum chloride is 25.49 gm