Given:
Initial temperature, T1 = 20.0 degrees Celsius
Final temperature, T2 = -1.5 degrees celsius
Energy, Q= 165 kJ
Specific heat capacity of the mixture, c = 3500 J/kg degrees Celsius
Sppecific latent heat, L = 255000 J/kg
Let's find the mass of the mixture.
Apply the formula:
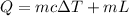
Where:
m is the mass
Rewrite the formula for m
Thus, we have:
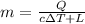
Now, input values and solve for m:
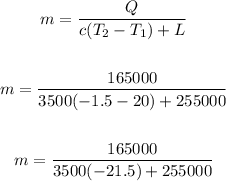
Solving further:
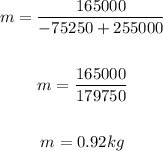
Therefore, the mass of the mixture is 0.92 kg