We have an equation (1) in equilibrium and we are given the value of its equilibrium constant Kc1 = 4.4x10^8.
The second reaction corresponds to the same initial reaction but now the coefficients are multiplied by two. Let us determine the Kc equations for each reaction.
Reaction 1:
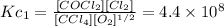
Reaction 2:
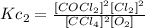
Now, if we compare both equations we can notice that the equation of the second reaction is equivalent to the first reaction squared, that is:
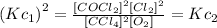
Therefore, the value of Kc for the second reaction will be:
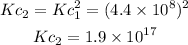
Answer: Kc for the reaction 2CCl4 + O2 <--->2COCl2 + 2Cl2 is 1.9 x 10^17