Answer:
98.079grams
Explanations:
The formula for calculating the moles of H2SO4 required is expressed as:
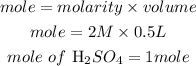
Calculate the mass of H2SO4
Mass = mole * molar mass
Mass = 1 mole * 98.079g/mol
Mass of H2SO4 = 98.079 grams
Hence the mass of H2SO4 needed to make 0.5 L of a 2M solution of H2SO4 is 98.079grams