Answer:
The minimum voltage that will be necessary to drive the reaction is -0.89V.
Step-by-step explanation:
- In the drawing we can see the electrolytic cell in which the reduction of Mn occurs at the cathode, and the oxidation of Sn occurs at the anode, that are connected by a salt bridge (color green in the image).
- To calculate the minimum voltage of the cell, it is necessary to write the half-reactions with their standard potencials (and turn it around in the case of Sn, because the standard potencial tables have reduction reactions), and the write the overall reaction:
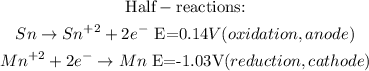
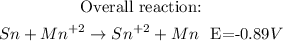
So, the minimum voltage that will be necessary to drive the reaction is -0.89V, and the negative sign means that the reation will take place spontaneously in reverse of how it is written.