Answer
44.77%
Step-by-step explanation
Given:
Mass of H₂O₂ decomposed = 156 g
Actual yield of O₂ = 23 L
Equation: 2H₂O₂ → O₂ + 2H₂O
What to find:
The percent yield of O₂.
Step-by-step solution:
To get the percent yield of O₂, you need to first calculate the theoretical yield of O₂.
From the given equation, 2 mol of H₂O₂ produced 1 mol of O₂
Note: 1 mol H₂O₂ = 34.0147 g H₂O₂, so 2 mol H₂O₂ = 2 x 34.0147 g = 68.0294 g
Also, 1 mol O₂ = 22.4 L
So we can say, 68.0294 g H₂O₂ produced 22.4 L O₂
Therefore, 156 g H₂O₂ will produce:
![\frac{156\text{ g H^^^^2082O^^^^2082 }*22.4\text{ L O^^^^2082}}{68.0294\text{ g H^^^^2082O^^^^2082}}=51.37\text{ L O^^^^2082}]()
Hence, the theoretical yield of O₂ = 51.37 L
Now, the percent yield of O₂ can be calculated using the formula below:
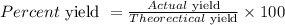
Actual yield of O₂ = 23 L
Theoretical yield O₂ = 51.37 L
So the percent yield of O₂ is:
![\begin{gathered} \%\text{ yield of O^^^^2082 }=\frac{23\text{ L}}{51.37\text{ L}}*100 \\ \%\text{ yield of O^^^^2082 }=0.447732139*100 \\ \%\text{ yield of O^^^^2082 }=44.77\% \end{gathered}]()
Hence, the percent yield of O₂ is 44.77%