Molality is a measure of concentration that relates the moles of solute to the kilograms of solvent, it is described by the following equation:
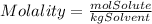
We are given the molality(2.50m) and kilograms of solvent(0.500kg), so we solve for moles of solute from the equation:
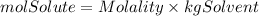
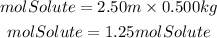
To make a 2.50molal NaOH solution would be needed 1.25moles of solute
Answer: 1.25 moles