Answer
(d) 5.12 g O₂
Step-by-step explanation
Given:
Mass of oxygen = 25.0 g
Equation: 4 K(s) + O₂(g) → 2K₂O(s)
From the balanced equation for the reaction, 4 moles of K react with 1 mole of O₂.
Note that from the Periodic Table, 1 mole of K = 39.10 g and 1 mole of O₂ = 32.00 g
In grams, 4 x 39.10 g = 156.40 g K reacts with 32.00 g O₂
So 25.0 g K will react with:
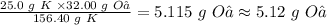
Therefore, 5.12 grams of O₂ are needed to react with 25.0 g of K