Answer:
1 mole of HCl
Explanations
The balanced chemical reaction between the antacid sample (NaOH) and hydrochloric solution (HCl) is given as;
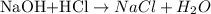
From the balanced reaction, we can see that 1 mole of HCl reacted with 1 mole of the antacid sample according to the stoichiometry ratio.
Hence we can conclude that 1 mole of HCl reacted with the antacid sample during the chemical reaction