Answer
C. 36.02
Step-by-step explanation
Given:
Moles of hydrogen that reacts = 2 mol
Moles of oxygen that reacts = 1 mol
What to find:
The mass of water formed when the moles of hydrogen and oxygen above react.
Solution:
The first step is to write a balanced chemical equation for the reaction to know the mole ratio of H to O
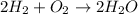
The mole ratio of hydrogen to water in the balanced equation is 2:2
The next step is to calculate the mole of water produced from the given moles of H and O using the mole ratio.
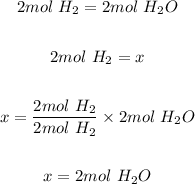
The final step is to convert the 2 moles of water produced to mass in grams using the molar mass of water and mole formula.
The molar mass of water = 18.01g/mol
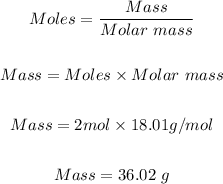
Hence, the mass of water formed is 36.02 g.
The answer is C. 36.02