Given:
The mass of the copper cylinder is: m = 76.8 g = 0.0768 kg
The change in the temperature is: T = 86.5 deg C - 19.5 deg C = 67 deg C
The specific heat is: c = 0.092 cal/g.C
To find:
Heat energy needed to heat the copper cylinder.
Step-by-step explanation:
The specific heat is defined as the amount of heat energy needed to raise the temperature of a substance by one degree celcius.
The expression relating heat Q, mass m, specific heat c and temperature difference T is:
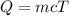
Substitute the values in the above equation, we get:
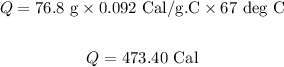
Final answer:
473.40 calories of heat is required to heat the copper cylinder.