The first step we have to follow to answer this question is to find the molar mass of the empirical formula, to do it add the atomic masses of carbon and hydrogen:
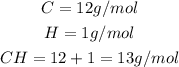
It means that the empirical formula has a mass of 13g/mol.
The next step is to divide the molar mass of the compound by the molar mass of the empirical formula:
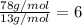
Finally, multiply the subscripts of the empirical formula by the result of the division.
It means that the molecular formula is:
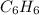
It means that the correct answer is d. C6H6.