INFORMATION:
We have the next balanced equation
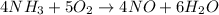
And we must find how many grams of NO are produced if 17 moles of O2 are reacted
STEP BY STEP EXPLANATION:
To find how many grams of NO are produced if 17 moles of 02 are reacted, we must analyze the equation.
We can see in the equation that:
- 1 mole of NH3 (on complete reaction) gives 1 mole NO.
- 5 moles of O2 produce 4 moles of NO, so 1 mol of O2 gives 4/5 = 0.8 mole of NO.
Thus, O2 will be the limiting reactant.
Now, if 17 moles of O2 are reacted, we must calculate the number of moles of NO that will be produce multiplying 0.8 by 17
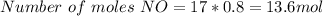
Then, using the molecular mass of NO (30.01 g/mol), we can calculate the number of grams that are produced
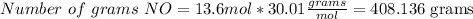
ANSWER:
408.136 grams of NO are produced if 17 moles of O2 are reacted.