The question requires us to calculate the mass of 6.30 moles of Ca(OH)2.
To calculate the mass of the given number of moles of Ca(OH)2, first we need to calculate the molar mass of this compound and then multiply it by the number of moles given.
- Calculating the molar mass:
We calculate the molar mass from the atomic mass of the elements Ca, O and H (the elements present in the molecule) and considering the number of atoms of this elements.
The atomic masses are:
atomic mass (Ca) = 40.08 u
atomic mass (O) = 15.99 u
atomic mass (H) = 1.01 u
There is 1 atom of Ca, 2 atoms of O and 2 atoms of H in the molecule. Thus, we calculate the molar mass of Ca(OH)2 as:
molar mass Ca(OH)2 = (1 * 40.08) + (2 *15.99) + (2 * 1.01) = 74.08 g/mol
- Calculating the mass of 6.30 moles of Ca(OH)2:
Now that we have the molar mass of Ca(OH)2, we can use it to calculate the mass of the given number of moles of this compound. To do that, we'll use the following equation:
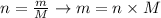
where n is the number of moles, m is the mass and M is the molar mass.
The calculation we need to do, with units, is as it follows:
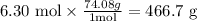
Therefore, the mass of 6.30 moles of Ca(OH)2 is 466.7 g.