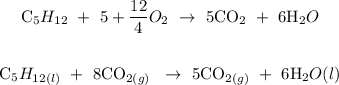ANSWER
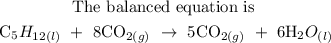
Step-by-step explanation
When an organic compound is burns in air, the type of reaction is called a combustion reaction.
The major products formed when an organic compound undergoes a combustion reaction are carbon dioxide and water.
To write a balanced equation for the reaction, follow the steps below
The combustion formula is written below as
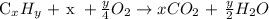
The compound given is pentane because it has 5 carbon atoms