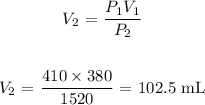Answer:
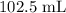
Step-by-step explanation:
Here, we want to get the final volume of the gas
According to Boyles' law, the volume is inversely proportional to the pressure
Mathematically:
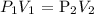
where:
P1 is the initial pressure of the gas which is 410 ml
V1 is the initial volume of the gas which is 380 mmHg
P2 is the final pressure of the gas which is 2 atm (we convert this to mmHg by multiplying by 760 mmHg: we have that as 2 * 760 mmHg = 1520 mmHg)
V2 is the final volume of the gas which is what we want to calculate
Substituting the values: