Answer:
1.7 g.
Step-by-step explanation:
What is given?
Mass of methane (CH4) = 1.1 g.
Mass of oxygen (O2) = 3.1 g.
Molar mass of CH4 = 16 g/mol.
Molar mass of O2 = 32 g/mol.
Molar mass of H2O = 18 g/mol.
Step-by-step solution:
First, let's state the balanced chemical equation:
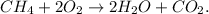
Now, let's convert the mass of each reactant to moles using their respective molar mass.
For CH4:
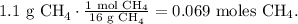
And for O2:
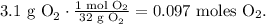
Now, let's see how many moles of H2O can be produced by each reactant.
You can see in the chemical equation that 1 mol of CH4 reacted produces 2 moles of H2O:
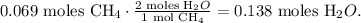
And you can see in the chemical equation that 2 moles of O2 reacted produces 2 moles of H2O. The molar ratio between these two substances is 1:1, which means that 0.097 moles of O2 reacted produce 0.097 moles of H2O:
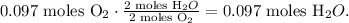
The limiting reactant, in this case, is O2 because this substance imposes the 'limit' and it's being consumed first, so actually, we're producing 0.097 moles of H2O.
The final step is to calculate the mass of H2O using its molar mass like this:
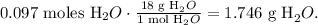
The answer would be that the theoretical yield of water (H2O) is 1.7 g.