Answer
× 10²³ molecules are in 41.8 g of sulfuric acid
Step-by-step explanation
The first step is to convert 41.8 g of sulfuric acid to moles by dividing the mass of sulfuric acid by its molar mass.
Molar mass of sulfuric acid, H₂SO₄ = 98.079 g/mol
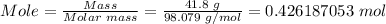
Finally, convert the moles of sulfuric acid to molecules using Avogadro's number.
Conversion factor: 1 mole of any substance = 6.022 × 10²³ molecules.
Therefore, 0.426187053 moles of sulfuric acid is equal
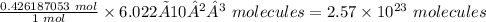
Thus, 2.57 × 10²³ molecules are in 41.8 g of sulfuric acid.