1) Write the chemical equation.
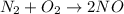
2) List the known and unknown quantities.
Sample: Oxygen (O2).
Pressure: 722 mmHg.
Temperature: 41 °C.
Sample: Nitric oxide (NO).
Amount of substance: 20.0 mol NO
3) Moles of oxygen to produce 20.0 mol NO.
The molar ratio between O2 and NO is 1 mol O2: 2 mol NO.
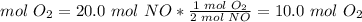
4) Volume of oxygen required.
4.1- Convert mmHg to atm.
1 atm = 760 mmHg
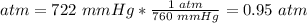
4.2- Convert °C to K.
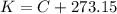
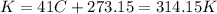
4.3- Set the equation (ideal gas equation)
Ideal gas constant: 0.082057 L * atm * K^(-1) * mol^(-1)
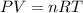
4.4- Plug in the know quantities and solve for V (liters).
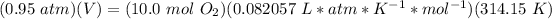
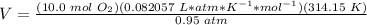
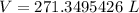
The volume of oxygen (O2) required is 271 L.