Answer:
pH = 2.6
pOH = 11.4
Explanations:
The formula for calculating the pH of a solution is given as;
![pH=-log[H^+]](https://img.qammunity.org/2023/formulas/chemistry/college/awlfu4vc4iesv0xee9hm70iq5p6gwfz7kw.png)
Given the following parameter
![[H^+]=2.5*10^(-3)](https://img.qammunity.org/2023/formulas/chemistry/college/8zqu2y5o6vb5z4o4lt2lpksbt48n2frdhc.png)
Substitute the given parameter into the formula
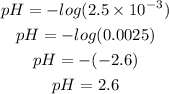
Determine the pOH of the solution
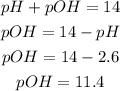
Therefore the pH and pOH of the solutionare 2.6 and 11.4 respectively