INFORMATION:
We must determine what is the effect of raising the temperature in an endothermic reaction
STEP BY STEP EXPLANATION:
To determine it, we need to know that:
If the system becomes hotter as the written reaction occurs from left-to-right (the forward reaction), the reaction is said to be exothermic. Conversely, if the system becomes colder as the forward reaction occurs, the reaction is said to be endothermic.
Now, We have the endothermic reaction at equilibrium:
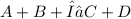
Where the Δ symbol represents energy in.
In this case, the reaction is endothermic and, an increase in temperature will cause the forward reaction to occur, increasing the amounts of the products and decreasing the amounts of reactants.