Answer:
Explanations:
Beta decay is a type of radioactive decay in which a beta particle is emitted from the nucleus. A beta particle is written as:
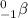
The required balanced nuclear equation for the beta decay of Pb-212 will be expressed as:
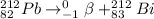
From the reaction, you can see that the beta decay of Pb-212 produces the Bismuth element with atomic number 83 and mass number 212.