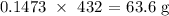Answer:
Step-by-step explanation:
Here, we want to get the mass of silver(I) dichromate needed
Firstly, we need to get the number of moles of Tin(I) Hydrogen Carbonate used up
To get this, we have to divide the mass used up, by the molar mass
The molar mass of Tin(I) Hydrogen Carbonate is 241 g/mol
Mathematically:
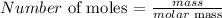
We have the number of moles as:
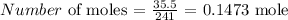
From the equation of reaction, 1 mole of Tin hydrogen carbonate used 1 mole of silver dichromate
That means 0.1473 mole of the carbonate will also use 0.1473 mole of the dichromate
Now, to get the mass of the dichromate, we have to multiply the number of moles by the molar mass of the dichromate
The molar mass of the dichromate is 432 g/mol
Thus, we have the mass used up as: