The first step we have to follow is to balance the given equation, making the number of atoms of each element equal in both sides of the equation.
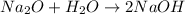
Now, that the equation is balanced, we can use the stoichiometric ratio of Na2O to NaOH to find the number of moles of NaOH produced from 1.20moles of Na2O:
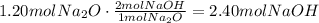
2.40 moles of NaOH are produced.