First, let's put the info into symbols:
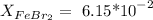
As the mole fractions have to add up to 1, we can calculate the water mole fraction, substracting:
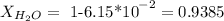
If we make the supposition that we have 1 mole of the solution, we would have the next quantities of each substance:
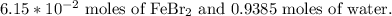
Now, we can convert these moles into mass through the molecular weight of each substance:
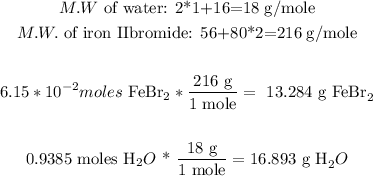
Now, we can calculate the mass percentage, because we have the mass of the substrate and the mass of the solution (the addition of water and iron (II) bromide):
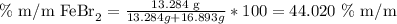
The answer is that the %m/m of iron bromide in the solution is 44.020%