wThe question requires us to define the ways the volume of a baloon can be increased, considering number of molecules of air, temperature and pressure.
To answer this question, we must analyze the ideal gas law, which can be written as:
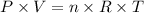
where P is the pressure of the system, V is its volume, n is the number of moles, R is a constant (constant of gases) and T refers to the temperature of the system.
We can rearrange this equation to analyze the effect of P, n and T over V:
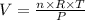
Looking at the equation, we can note that temperature (T) and number of moles (n) are directly proportional to volume (V), while pressure (P) is inversely proportional.
This means that increasing / decreasing the number of moles or the temperature of the system will increase / decrease the volume, while increasing the pressure will decrease the volume (and decreasing the pressure will increase volume):
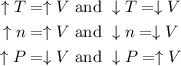
Considering the information above, we can answer the question as it follows:
• increasing the number of air molecules in the balloon
• increasing the temperature of the balloon
• decreasing the pressure on the balloon