To calculate the number of moles we will use Avogadro's number, which relates the number of molecules contained in a mole of any substance, the relationship between moles and molecules is as follows:
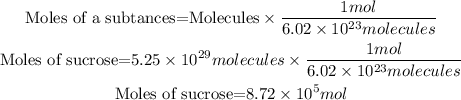
In the statement we are given the molecules with 3 significant figures, therefore the answer must also have 3 significant figures. So the answer will be: In 5.25x10^29molecules are 8.72x10^5 mol of sucrose