Answer: 45g of NO2 are produced (third option or letter C).
Step-by-step explanation:
The question requires us to calculate the amount of NO2, in grams, that would be obtained from 250 mL of 2.6 M HNO3 and excess of NO in the following chemical reaction:
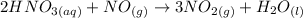
To solve this problem, we must calculate the number of moles of HNO3 used, considering the volume and solution concentration given, then use the stoichiometry of the reaction to calculate the amount of moles of NO2 produced and, at last, use the molar mass of NO2 to convert the number of moles to mass.
Considering the definition of molarity, we can write:
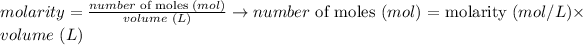
Thus we need the volume of HNO3 solution used (250 mL = 0.250 L) and the molarity of the solution (2.6 M) to calculate the number of moles of HNO3 used in the reaction:
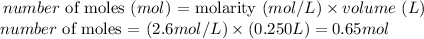
Therefore, 0.65 moles of HNO3 were used in the reaction.
Considering the stoichiometry of the reaction, given by the chemical equation, we can say that 2 moles of HNO3 are necessary to obtain 3 moles of NO2. Thus, we can write:
2 mol HNO3 --------------------- 3 mol NO2
0.65 mol HNO3 ---------------- x
Solving for x, we'll have:
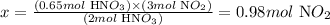
Therefore, 0.98 moles of NO2 are obtained when 0.65 moles of HNO3 are used.
Next, we need to use the molar mass of NO2 (46.00 g/mol) to calculate the mass that corresponds to 0.98 moles of NO2:
1 mol NO2 ---------------- 46.00g NO2
0.98 mol NO2 ---------- y
Solving for y, we'll have:
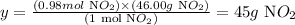
Therefore, 0.98 moles of NO2 correspond to 45g of this compound.
The best option to answer this question is the third one (or letter C): 45g.