ANSWER
he number of atoms of lithium is 5.039 x 10^24 atoms
Step-by-step explanation
Given that;
The number of moles of lithium is 8.37 mol
Follow the steps below to find the number of atoms of lithium
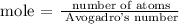
Recall, that the Avogadro's number is 6.02 x 10^23
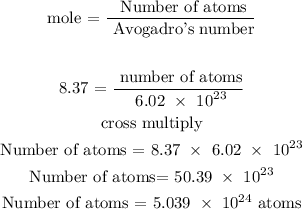
Therefore, the number of atoms of lithium is 5.039 x 10^24 atoms