INFORMATION:
We know that:
- An aqueous solution is 15.0% methanol (CH4O) by mass and has a density of 0.998 g/mL
And we must calculate molarity, molality and Xa
STEP BY STEP EXPLANATION:
First, we must analyze the given information:
15.0% methanol (CH4O) by mass means,
mass of methanol = 15 g
mass of water (solvent) = 85 g = 0.085 kg
mass of solution = 100 g
Now, we can calculate:
a. Molarity
To calculate it, we must use the following formula
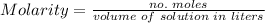
The solution has a density of 0.998 g/mL
Using that,
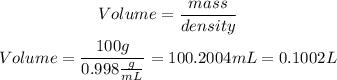
Now, calculating no. of moles
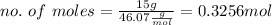
Finally, replacing the values in the formula for Molarity
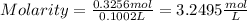
b. Molality
To calculate it, we must use the following formula
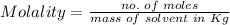
Now, replacing the values in the formula
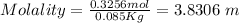
c. Xa (mole fraction)
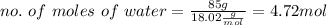
Now, the total moles would be
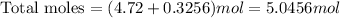
Then, the mole fraction
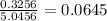
ANSWER:
a. 3.2495 mol/L
b. 3.8306 m
c. 0.0645