Step 1 - What is molar fraction?
The molar fraction of a substance can be obtained by dividing its number of moles by the total number of moles in the solution (i.e., moles of solute + solvent):
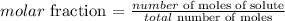
Step 2 - Calculating the molar fraction of urea
Let's calculate the number of moles for each substance in the solution (water and urea). In order to do so, we just have to divide their mass by their molar mass:
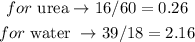
The molar fraction of urea can be writen thus as:
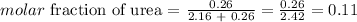
Answer: the molar fraction of urea in this solution is 0.11