Answer:
b. The pressure will be cut in half.
Step-by-step explanation:
As we can see in the Ideal Gases formula, the pressure and the number of moles are directly proportional:
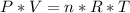
if the temperature and volume remain constant, when extracting 4 moles, the amount of substance inside the container decreases, that is, there are fewer particles, therefore the pressure will be lower.
When the number of moles is cut in half (from 8 moles to 4 moles), the pressure will also be cut in half.